Describe the Development of the Modern Periodic Table
Modern periodic table The modern periodic table consists of 7 horizontal periods and 16 vertical groups 18 vertical columns. As a result they react in the same way.

What Is The Heaviest Naturally Occurring Element On Earth Trivia Genius How To Memorize Things Periodic Table Chemistry
Order a custom-written plagiarism-free paper WhatsApp Order Now.

. Um and this starts to look a little bit more like our modern periodic table. Check all that apply. The work of John Newlands and Dmitri Mendeleev led to the development of the modern periodic table.
Describe how the elements belonging to a group of the periodic table are. Looking for a Similar Assignment. Describe the modern periodic table.
The history of the periodic table is a reflection of more than two centuries of growth to understand the chemical and physical properties of the elements. Explain how the periodic law can be used to predict the physical and chemical properties of elements. His original list showed only five elements.
Next we have Dmitri Mendeleev who was a Russian chemist and what he did is he arranged wth e elements based on the sequence of atomic mass. Include contributions made by Lavoisier Newlands Mendeleev and Moseley. Hydrogen oxygen azote nitrogen carbon and sulfur along with their atomic weights.
Describe the development of the modern periodic table. Explain the roles of Mendeleev and Moseley in the development of the periodic table. Group 1 consists of highly reactive metals.
Newlands was the first to organize the elements and show that properties repeated in a periodic way. The modern periodic table was devised by Dmitri Mendeleev and is a useful framework for organizing and analyzing chemical and physical behavior of the elements. Development of the periodic table.
Include contributions made by Lavoisier Newlands Mendeleev and Moseley. Describe the development of the modern periodic table. The elements of the modern periodic table are classified into 4 blocks s p d and f.
Therefore the bottom-left most element francium is predicted to have the lowest electronegativity and the top-right most element fluorine is predicted to have the highest electronegativity. Modern periodic table is based on modern periodic law which states that physical and chemical properties of elemnts are periodic function of their atomic numbers. Lavoisier organized a list.
Newlands was the first to organize the elements and show that properties repeated in a periodic way. Include contributions made by Lavosier Newlands Mendeleev and Moseley. Seaborg Dmitri Mendeleev and.
In the 1860s scientists began to try to sort the known elements into a logical sequence. Describe the development of the modern periodic table. In the 1860s scientists began to try to sort the known elements into a logical sequence.
Mendeleevs periodic table also organized the elements by increasing atomic mass became the 1st widely accepted organization scheme for the elements. The main-sequence of elements within a periodic group have the same number of valance electrons. Lavoisier organized a list of the known elements of his day as four categories.
The groups are labelled 1 2 transition metals 3 4 5 6 7 and 0 or 8. The modern periodic table lists the elements in order of increasing atomic number the number of protons in the nucleus of an atom. The columns of the modern periodic table are called groups or families.
The notation in the periodic table includes references to atomic mass and atomic number. The periods in the modern periodic table The periods include the elements of the different properties and they have. 1 on a question.
People also asked Study Guides. Mendeleev produced the first orderly arrangement of known elements. The major contributions to the development of the periodic table were made by Antoine-Laurent de Lavoisier John Newlands Johann Wolfgang Döbereiner Julius Lothar Meyer Glenn T.
Um and this starts to look a little bit more like our modern periodic table. Include contributions made by Lavoisier Newlands Mendeleev and Moseley. In the modern periodic table the electronegativity of elements increases across a period row and decreases down a group column.
The work of John Newlands and Dmitri Mendeleev led to the development of the modern periodic table. 1 2 3 4 5 6 7 0 transition metals All the elements in a group have similar chemical properties eg. Chemists have always looked for ways of arranging the elements to reflect the similarities between their properties.
Include contributions made by Lavoisier Newlands Mendeleev and Moseley. Next we have Dmitri Mendeleev who was a Russian chemist and what he did is he arranged wth e elements based on the sequence of atomic mass. John Daltons Periodic Tables In 1803 the English school teacher and part-time scientist John Dalton published his first list of elements when he printed his atomic theory and his early gas law work.
Describe the development of the modern periodic table. Which statements accurately describe Dmitri Mendeleevs contributions to the development of the periodic table. Lavoisier organized a list of the known elements of his day as four categories.
Development of the periodic table The periodic table is arranged in columns called groups. Mendeleev ordered the elements by increasing atomic number. Historically however relative atomic masses were used by scientists trying to organise the.
Newlands organized the elements by increasing atomic mass. Mendeleev wrote the first modern chemistry textbook. Describe the development of the modern periodic table.
Up to 24 cash back Chapter 5. Describe the development of the modern periodic table. Include contributions made by Lavoisier Newlands Mendeleev and Moseley.

Html5 Table Of Elements Periodic Table Of The Elements Periodic Table Web Design

What Are The First 20 Elements Periodic Table Of The Elements Periodic Table Art Periodic Table Words

Chapter 6 The Periodic Table Physics Periodic Table Behavior

Periodic Table With 118 Elements Can Print Very Large For Decoration Chemistry Periodic Table Science Notes Periodic Table Of The Elements

Biography Of Dmitri Mendeleev Inventor Of The Periodic Table Dmitri Mendeleev Periodic Table Scientific Breakthroughs

The Periodic Table Of Digital Marketing The Ultimate Chart Of Elements For The Digital Era Digital Marketing Periodic Table Marketing

3 2 Development Of The Modern Periodic Table Chemistry Libretexts Periodic Table Of The Elements Periodic Table Element Chemistry

Development Of The Periodic Table Introduction To Chemistry

3 2 Development Of The Modern Periodic Table Chemistry Libretexts
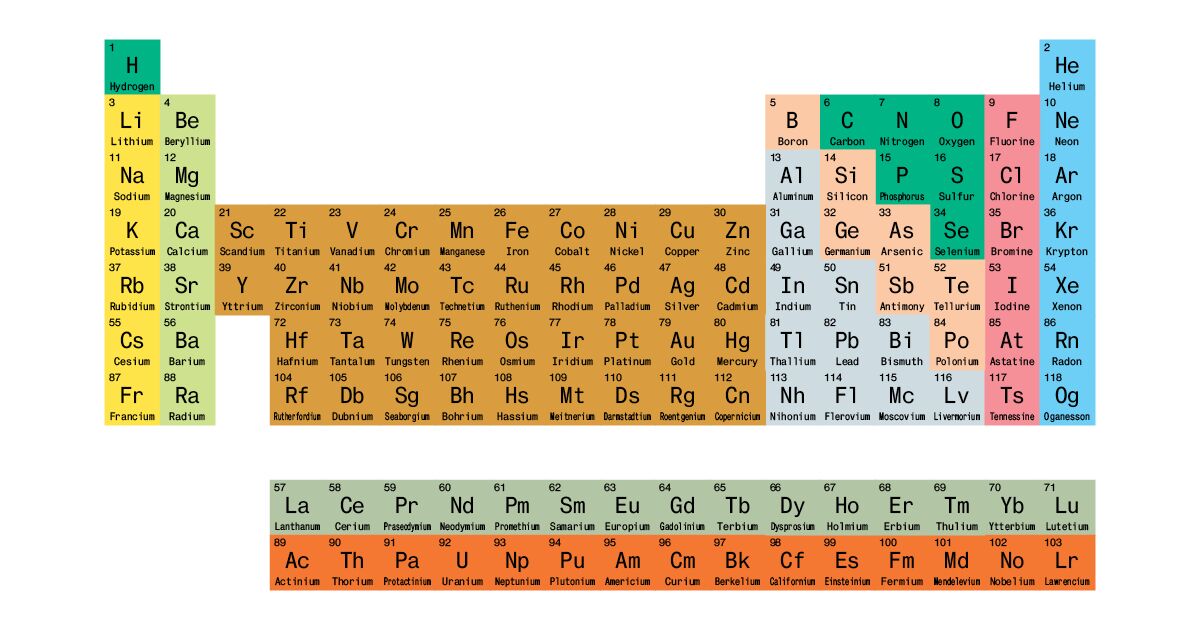
The Modern Triumph Of The Periodic Table Of Elements Bloomberg

Periodic Properties Of The Elements Chemistry Basics Chemistry Education Teaching Chemistry
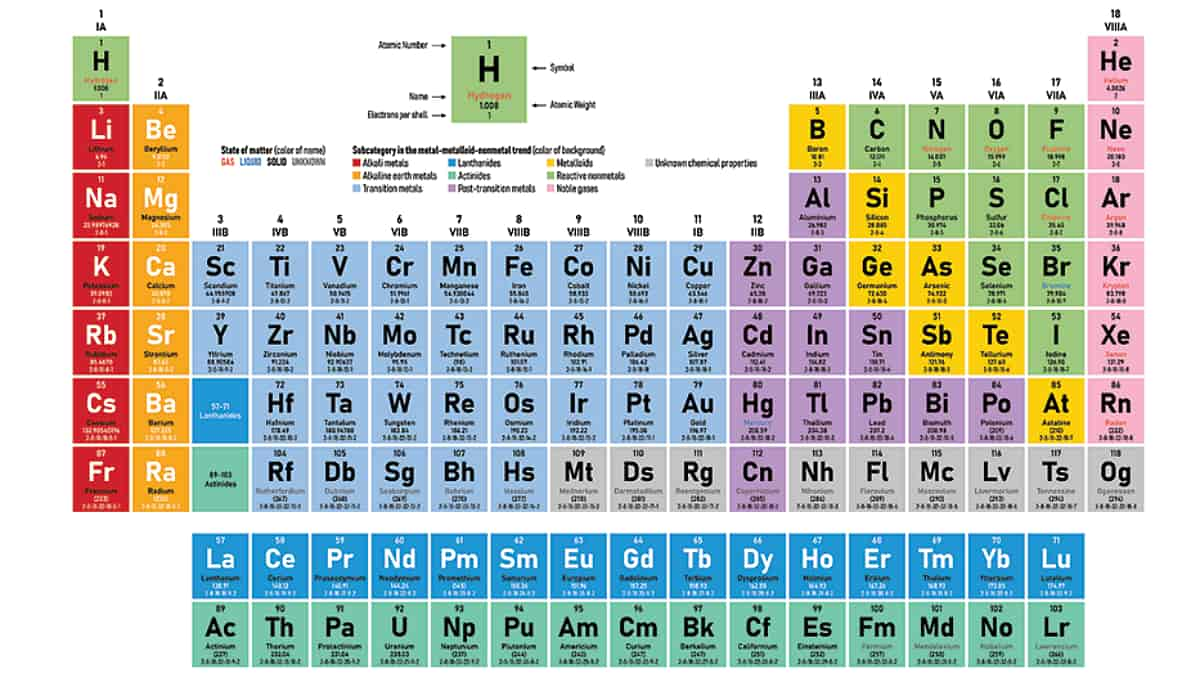
National Periodic Table Day 2022 History Facts And All You Need To Know About The Periodic Table

Bell Ringer 1 What Are The Properties Of Metals 2 What Are The Properties Of Non Metals Bell Ringers Periodic Table Chapter

This Is A Periodic Table With Atomic Mass Element Name Element Symbol And Atomic Num Periodic Table Periodic Table With Names Periodic Table Of The Elements
Timeline Development Of The Modern Periodic Table By Colby Rookstool

Describe The Contribution Of John Newlands In The Development Of The Modern Periodic Tabl Periodic Table Of The Elements Element Chart Periodic Table Printable


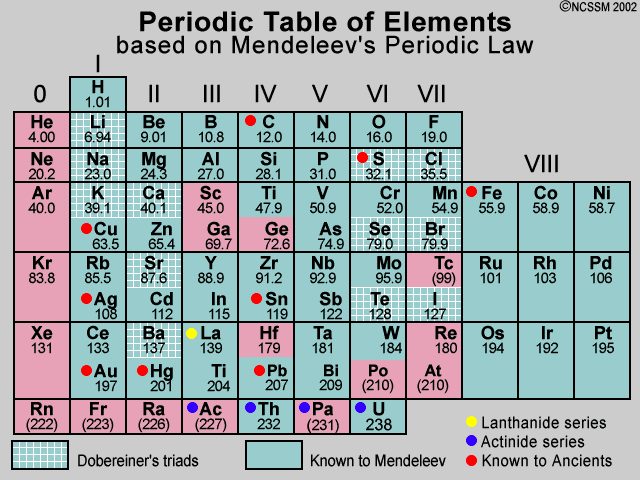
Comments
Post a Comment